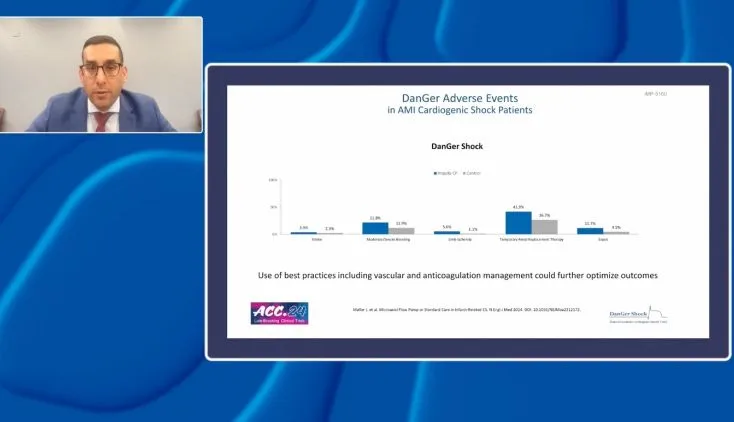
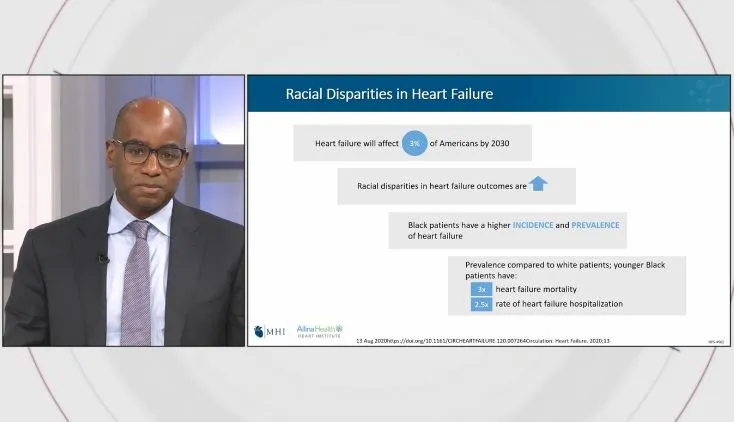
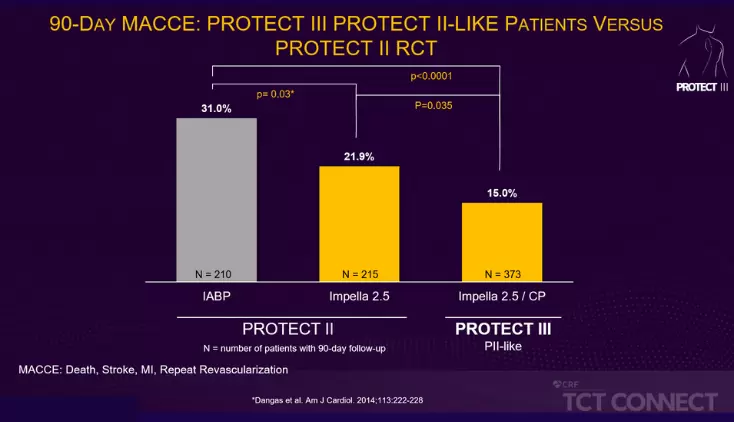
Impella Education, Patient Management
Impella® Skills Video: Guidewire Reaccess
This Impella Skills video demonstrates how to use the repositioning sheath with guidewire access port on the Impella CP® or Impella CP® with SmartAssist® to insert a 0.035” guidewire to maintain access to the arteriotomy. The stylet within the access port maintains patency and should not be removed from the port until access is needed.
- Loosen and detach the anticontamination sleeve.
- Remove the stylet from the sidearm of the repositioning sheath hub.
- Aspirate the port to confirm arterial blood flow, which indicates that the tip of the sheath is inside the artery and the line is patent.
- Flush the port.
- Under fluoroscopic guidance, insert a 0.035” J-tip guidewire through the sidearm. If you encounter any difficulty advancing the guidewire, rotate the sidearm to reorient the direction of the wire exiting the sheath tip.
- Advance the guidewire into the descending aorta, above the level of the renal arteries.
- Pull the Impella catheter back across the aortic valve at P-level 1 (P-1).
- Turn the Impella off by reducing flow to P-0.
- Pull the catheter back until it reaches the distal end of the repositioning sheath.
- Remove the Impella device and repositioning sheath from the arteriotomy, leaving the wire in place.
- Immediately apply pressure to the access site to control bleeding.
- If replacing the Impella device, insert the peel-away sheath provided with the new Impella Setup & Insertion kit and follow insertion steps to insert the new device.
- If device replacement is not required, insert an appropriately sized sheath over the 0.035” guidewire, ensuring that hemostasis is maintained.
This guide is intended to be an educational tool and does not replace the Instructions for Use (IFU) manual.
IMP-2533