Clinical Research & Data, Safety & Efficacy, AMI Cardiogenic Shock
FAQ: Is There Randomized Controlled Trial Data Available for Impella® Heart Pump Use in Cardiogenic Shock?
Randomized controlled trials (RCTs) are considered the “gold standard” for evaluating the efficacy and safety of medical interventions. Abiomed is committed to providing the best possible clinical care and evidence of the beneficial effect of Impella in critically ill patients with cardiogenic shock. To date, seven randomized trials have been attempted with Impella and five were discontinued following inadequate enrollment to evaluate the primary outcome of survival benefit with Impella compared to control intervention.
The ISAR-SHOCK trial comparing the hemodynamic effects of Impella with IABP in acute myocardial infarction and cardiogenic shock (AMICS) was completed. The RECOVER II trial was planned to evaluate patients with AMICS but was discontinued after 18 months following the enrollment of only one patient from 50 IRB-approved sites. The DANSHOCK trial enrolled 100 patients with AMICS in Denmark in its initial five years. It was recently expanded to enroll patients from Germany and renamed the DanGer Shock trial. It is actively enrolling with a target of 360 patients.
Additionally, in September 2022, Abiomed announced the FDA has approved the Abiomed-sponsored RECOVER IV RCT. RECOVER IV will compare outcomes in AMICS patients who receive Impella support pre-PCI to those who receive conventional therapies, including any type of mechanical circulatory support other than Impella.
Randomization in Cardiogenic Shock is Challenging for Ethical & Logistical Reasons Without Exception from Informed Consent (EFIC)
FRENCH Trial
The FRENCH trial was a multicenter randomized trial comparing standard treatment to standard treatment plus extracorporeal life support (ECLS) or the Impella 2.5® heart pump in patients with AMI cardiogenic shock (AMICS).1 Standard treatment included use of intra-aortic balloon pump (IABP), inotropic drugs and antiplatelet agents based on the recommended treatment at the study hospital. The primary endpoint was all-cause mortality at 30 days or evolution to refractory cardiogenic shock requiring left ventricular assist device (LVAD).
The sample size required to detect differences in the primary outcome was 200 patients; however, only 19 patients were enrolled over 52 months resulting in the discontinuation of the study due to low enrollment rate. The results of this trial are not publicly available.
ISAR-SHOCK
ISAR-SHOCK was intended to be a prospective, randomized trial, but had non-randomized execution. It compared the hemodynamic effects of the Impella heart pump with the effects of the intra-aortic balloon pump (IABP).2 Over the course of 19 months, 26 patients with AMI for less than 48 hours and cardiogenic shock were assigned IABP or Impella 2.5 support after percutaneous coronary intervention (PCI). At baseline, median left ventricular ejection fraction (LVEF) was 27.5% and the time from AMI onset was 4.5 hours with no significant difference in baseline characteristics observed between groups. The primary endpoint was improvement in cardiac index 30 minutes after device implantation.
IABP was safely placed in 13 patients and Impella 2.5 in 12 patients. One patient randomized to Impella 2.5 died before implantation. The cardiac index after 30 minutes of support increased significantly with Impella 2.5 compared to IABP (0.49 ± 0.46 L/min/m2 vs. 0.11 ± 0.31 L/min/m2, p = 0.02). In addition, the diastolic arterial pressure increased with Impella compared to reduction with IABP support (9.2 ± 12.1 mmHg vs. -8.0 ± 13.1 mmHg, p = 0.002). The secondary endpoint of all-cause mortality at 30 days was 46% in both groups. Higher rates of hemolysis were observed in patients with Impella in the first 24 hours requiring more transfusion. No device-related malfunction, major bleeding or ischemic events were reported during support with Impella or IABP. A trend toward a more rapid reversal of serum lactate levels was observed in patients with Impella compared to IABP.
IMPRESS in STEMI Trial
The purpose of this multicenter, international randomized trial was to compare intra-aortic balloon pump (IABP) and the Impella 2.5 heart pump after percutaneous coronary intervention (PCI) in the setting of cardiogenic pre-shock anterior STEMI.3,4 Cardiogenic pre-shock was defined as heart rate >100 beats/min and/or systolic blood pressure <100 mmHg with clinical signs of cold extremities, cyanosis, oliguria, and decreased mental status. The primary endpoint was left ventricular ejection fraction (LVEF) assessed by MRI at four months follow-up.
The target number of participants to assess the primary outcome of this study was 130 patients; however, only 21 patients were randomized to Impella (n = 12) or IABP (n = 9) in five centers over 42 months. Due to the small number of patients enrolled, the trial was stopped prematurely, precluding an appropriate assessment of clinical outcomes.
RECOVER II FDA Trial
RECOVER II was a multicenter, open-label, randomized trial initiated to compare clinical outcomes with the Impella 2.5 heart pump and the intra-aortic balloon pump (IABP) in patients with AMI cardiogenic shock (AMICS).6 The primary outcome was composite rate of major adverse events within 30 days or at hospital discharge and the secondary outcome was maximum increase in cardiac power output from baseline. The sample size needed to determine significant differences between groups in major adverse events was 384.
Despite 50 sites with IRB approval in the US, only one patient was enrolled in the study between July 2008 and August 2010, resulting in discontinuation of the trial due to low enrollment.
IMPRESS Trial
The IMPRESS trial was an open-label trial initiated to determine whether the Impella heart pump can decrease 30-day all-cause mortality compared to IABP in patients with severe shock complicating acute myocardial infarction.8 Based on the assumption that survival in severe shock is less than 10%, the sample size needed to determine significant differences in mortality between groups was initially determined to be 48 (24 patients in each group). At the interim analysis, it was evident that the mortality in the control group was much lower than original assumption of 90%. Since >100 patients would be required to determine a difference in mortality between Impella and IABP, it was decided to complete the study with 48 patients as an exploratory analysis.
Given that the trial was statistically underpowered, no difference in mortality was observed between groups at 30 days and 6 months. Mortality at 30 days was 46% in patients treated with Impella compared to 50% with IABP (p = 0.92). At six months, mortality rate was 50% in both treatment groups (p = 0.92). Importantly, about 92% of the enrolled patients had cardiac arrest with 48% having return of spontaneous circulation (ROSC) longer than 20 minutes. All patients were treated with catecholamines before randomization and 75% received therapeutic hypothermia. Of the 24 patients in either intervention group, about 84% had device placement after revascularization. In addition, overall, 15% of patients had traumatic injuries due to cardiac arrest with disproportionately higher rates observed in the Impella group compared to the IABP group (21% vs. 8%). The main cause of death was brain damage and refractory cardiogenic shock in 46% and 29%, respectively.
Many shortcomings are identified in the conduct of the trial, which had non-randomized or sequential execution. The definition used for severe cardiogenic shock is unclear and patients were deemed to have cardiogenic shock based on operator discretion. As per protocol, crossover between treatments was not allowed; however, three patients in the IABP group crossed over to Impella group. Among the patients in the Impella group, one patient received Impella 5.0® after Impella CP®, one patient received IABP before Impella thus constituting a protocol violation, and one patient did not receive Impella after randomization.
Major bleeding was reported in 33% of patients in the Impella arm compared to 8% in the IABP arm. Given the disproportionately higher rates of traumatic injuries at admission in patients receiving Impella, higher rates of bleeding events are not unexpected. In fact, device-related bleeding events were not different between groups with three events in the Impella group and one event in the IABP group.
In the combined analysis of the entire study population, a trend toward lower mortality at 30 days was observed if the mechanical circulatory support device was used pre-PCI (25% vs 53%, p = 0.16). Similar trends of lower mortality were observed in patients with ROSC <20 minutes and lactate levels lower than 7.5mmol/L at admission. Given the high rates of post-anoxic neurological damage, the use of any mechanical circulatory support device may be of limited utility.
While this study offers insight into the use of Impella in patients with cardiac arrest, it does not address the survival outcome associated with the use of Impella in AMICS. Hence, it is better to describe this trial as ‘IMPRESS in Cardiac Arrest (CA).'
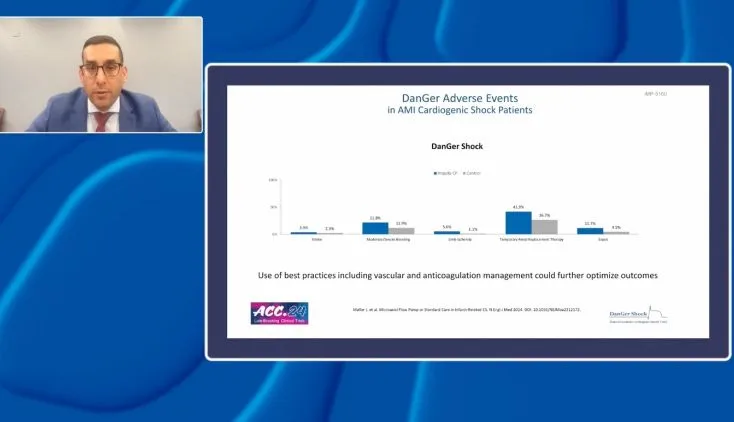
DanGer Shock Trial
The DanGer Shock trial is an ongoing, open-label randomized trial initiated in patients with AMI cardiogenic shock (AMICS) in Denmark7 and given the name DanGer Shock trial when it was expanded to enroll patients from Germany. A total of 360 patients are planned to be enrolled to assess the primary outcome of death from all causes at six months with the Impella CP heart pump compared to standard care. Inclusion criteria for study participants include STEMI for <36 hours, cardiogenic shock for <24 hours confirmed based on arterial blood lactate ≥2.5mmol/L and/or SvO2 <55% with a normal PaO2 and systolic BP <100 mmHg and/or need for vasopressor therapy, and LV ejection fraction <45%.
Enrollment is ongoing. Since the study initiation in December 2012, >250 patients have been enrolled.
What are the challenges with conduct of randomized trials in cardiogenic shock?
Randomized trials play an important role in assessment of cardiovascular and medical device innovations, although they also pose many logistical and methodological challenges, particularly in the emergent setting of acute myocardial infarction and cardiogenic shock (AMICS). Consequently, evidence on outcomes of the Impella heart pump in AMICS based on randomized controlled trials is limited.
Variable definition of cardiogenic shock
Cardiogenic shock represents a continuum of patient condition ranging from hypotension after AMI to out of hospital cardiac arrest requiring multiple inotropes.9 It is therefore challenging to differentiate patients with cardiogenic shock who have not passed the window of opportunity for treatment from patients in the “hemo-metabolic state” who may have irreversible organ injury despite normalization of hemodynamic parameters such as cardiac arrest with anoxic brain injury.
Moreover, the adaptation of treatment options depending on the severity of shock affects both the inclusion criteria as well the clinical outcomes of the trial. While broadening inclusion criteria may increase the inclusion of patients in the trial, it might also mask the specific effect of the Impella heart pump in a subset of responder patient population. On the other hand, narrowing the inclusion criteria to exclude patients such as those with severe brain injury may help assess the impact of Impella but requires implementation of uniform protocols. Depending on the inclusion criteria, early identification and subsequent enrollment of eligible patients in the trial during emergency situations presents a significant challenge.
Low and slow enrollment
Despite repeated attempts at conducting randomized control trials with the Impella heart pump in patients with cardiogenic shock, the recurrent theme observed is both low and slow enrollment rates. Given the low incidence rate of cardiogenic shock of <10% of patients with AMI, the slower enrollment has been observed in randomized trials of mechanical assist devices.3 Moreover, patients with cardiogenic shock have high mortality and procedural risks due to presence of multiple comorbidities and extensive coronary disease and thus are not readily enrolled in clinical trials.10
Obtaining informed consent is a contributing factor to the low enrollment rates. Due to the emergent nature of intervention in the setting of myocardial infarction, seeking informed consent from the patient’s family may either cause additional delay or may not be possible at all. The exclusion of the study candidates most likely to benefit from Impella might also be due to a variety of circumstantial reasons such as availability of trained surgeons and individual preferences of treating physicians.
Choice of comparator and crossover issues
In a randomized trial, the choice of the comparator affects both the conduct and the interpretation of results. The intra-aortic balloon pump (IABP) is the most widely used comparator intervention in randomized trials of mechanical circulatory support devices in cardiogenic shock mainly due to relative familiarity and ease of use by interventional cardiologists.
However, randomization of patients to IABP despite clinical evidence pointing to lack of hemodynamic or mortality benefits poses an ethical dilemma and highlights the lack of clinical equipoise. The additional challenge encountered with management of patients randomized to “usual care” is crossover of patients who continue to deteriorate clinically and hemodynamically to the comparator arm. Such crossovers may affect the trial outcomes and the effect sizes of the interventions.
Outcome assessment
Due to the life-threatening nature of cardiogenic shock and the correlation of hemodynamic parameters with clinical outcomes, it is expected that mechanical circulatory support devices may affect the prognosis. Accordingly, the primary outcome of most randomized trials has been long- or short-term mortality. Calculating the sample size of the trials to detect significant difference in mortality outcomes likely contributes to underpowered study designs and inconclusive study results. A plethora of surrogate outcomes such as cardiac index, mean arterial pressure, lactate levels, and length of intensive care unit treatment have been previously assessed.
Not surprisingly, studies assessing hemodynamic benefits have reported significant improvements with Impella.2,9 Consensus on which outcomes are best assessed, when and how they should be measured is needed for randomized control trials in patients with cardiogenic shock.
Importantly, treatment of high-risk patients with cardiogenic shock involves a complex interplay of ‘systems of care’ in addition to use of Impella. Outcome of survival is dependent not only on the identification and appropriate use of Impella but also on the comprehensive care of patients during and after Impella support, which varies widely based on the patient’s underlying conditions and hospital practices.
Timing of Impella support
Given the importance of rapid revascularization, historically mechanical circulatory support devices were implanted after PCI. Contrary to established clinical practice, results of clinical studies with the Impella heart pump to date suggest early support with Impella prior to PCI and escalating doses of inotropes is associated with better clinical outcomes.9,11 In addition, beneficial effect of Impella in patients with futile situations such as severe brain injury appears to be unlikely. The years of clinical research have helped identify the optimal timing for implantation of Impella devices and have demonstrated that patients with shorter “shock to support” times are most likely to benefit with Impella use.11
What is the published clinical evidence for Impella use in cardiogenic shock?
Given the challenges with conduct of randomized trials in cardiogenic shock, the clinical outcomes after Impella heart pump support have been analyzed in real-world registries. The results from registries of Impella (cVAD Study, Impella Quality (IQ) Database) have consistently demonstrated good clinical outcome and safety in patients with AMI cardiogenic shock (AMICS) and informed the clinical community on best practices. Importantly, clinical evidence from studies on Impella has helped identify the optimal timing for implantation of Impella and has demonstrated that patients with shorter “shock to support” times are most likely to benefit with Impella use.11
Investigator-led studies that demonstrate Impella improves survival and native heart recovery in AMICS patients include:
BMJ Open Heart, 2020
A cohort study of all consecutive patients (n=903) with AMICS undergoing PCI <24 hours of symptom onset in southeastern Denmark from 2010 to 2017 comparing 30-day mortality between patients receiving early-IABP or early-Impella CP and their respective control group. That data showed early application of Impella CP was associated with reduced 30-day mortality compared with a matched control group.12
Frontiers Cardiovascular Medicine, 2020
Retrospective analysis of 166 consecutive IABP-SHOCK II-eligible cardiogenic shock patients in four dedicated shock centers that showed an observed mortality on circulatory support with an Impella was significantly lower than predicted in patients with highest mortality risk. Overall 30-day mortality was 42%, and mortality was higher in resuscitated patients (50 vs. 36%) and when Impella was implanted post-PCI (Impella-pre-PCI: 28%, Impella-post-PCI: 51%).13
Catheterization and Cardiovascular Interventions, 2021
Evaluation of data from 365 patients treated with Impella 2.5/Impella CP in 17 centers of the IMP-IT Registry with findings that suggest a survival benefit and reduced rates of major bleeding with pre-PCI Impella implantation instead of during or after the procedure. Pre-procedural insertion was associated with an improvement in one-year survival in AMICS patients treated with PCI and HR-PCI and early Impella support was associated with a lower rate of the composite of mortality, re-hospitalization for heart failure and need for left-ventricular assist device/heart transplantation at one-year.14
International Journal of Cardiology, 2022
This large meta-analysis of 13 studies (6810 patients) suggests that Impella placement prior to PCI in AMICS may have a positive impact on short- and midterm mortality compared with post-PCI, with similar safety outcomes. Short-term mortality was significantly reduced in those receiving pre-PCI vs. during/post-PCI Impella support (37.2% vs 53.6%), and midterm mortality was also lower in the pre-PCI Impella group (47.9% vs 73%).15
JSCAI, 2022
This study showed women had a 74% relative survival benefit with Impella placement pre-PCI compared to post-PCI highlighting that the benefit of Impella implantation pre-PCI is more prominent in women.16
National Cardiogenic Shock Initiative (NCSI) Study, 2021
This single-arm trial demonstrated a 71% survival to discharge with greater than 90% native heart recovery when best practices are used, including placement of Impella prior to PCI. The NCSI study evaluated outcomes of 406 patients at 80 community hospitals and academic medical centers in the U.S.
Journal of the American College of Cardiology, 2019
This study of 204 patients in the INOVA Health System showed an increase in survival at 30 days from 44% to 82% when utilizing a standardized team-based approach with a best practice protocol that includes early use of percutaneous MCS.17
Journal of Artificial Organs, 2022
This three-year interim analysis of all Impella patients treated in Japan found use of Impella was associated with a 77% AMICS survival rate at 30 days among a cohort at 109 hospitals. The study was conducted with oversight by 10 Japanese professional societies, including the Japanese Circulation Society.18
References
- Massetti, M. (2006). Comparison of Standard Treatment Versus Standard Treatment Plus Extracorporeal Life Support (ECLS) in Myocardial Infarction Complicated With Cardiogenic Shock. https://clinicaltrials.gov/ct2/show/NCT0314847?term=nct00314847&rank=1
- Seyfarth, M., et al. (2008). J Am Coll Cardiol, 52(19), 1584-1588.
- Ouweneel, D.M., et al. (2016). Int J Cardiol, 202, 894-896.
- Henriques, J.P. (2007). IMPRESS in STEMI.
- Griffith, B.P., et al. (2013). J Thorac Cardiovasc Surg, 145(2), 548-554.
- O'Neill, W.W. (2008). Trial Using Impella LP 2.5 System in Patients With Acute Myocardial Infarction Induced Hemodynamic Instability (RECOVER II). https://clinicaltrials.gov/ct2/show/nct00972270?term=nct00972270&rank=1
- Møller, J.E. (2012). Danish Cardiogenic Shock Trial (DanShock). https://clinicaltrials.gov/ct2/show/nct01633502?term=nct01633502&rank=1
- Ouweneel, D.M., et al. (2017). J Am Coll Cardiol, 69(3), 278-287.
- Basir, M.B., et al. (2018). Catheter Cardiovasc Interv, 91(3), 454-461.
- Rihal, C.S., et al. (2015). J Am Coll Cardiol, 65(19), e7-e26.
- Basir, M.B., et al. (2017). Am J Cardiol, 119(6), 845-851.
- Helgestad, OKL, et. al., (2020). Open Heart,7:e001214. doi: 10.1136/openhrt-2019-001214
- Schäfer, A., et. al., (2020). Front Cardiovasc Med, 7:74. doi: 10.3389/fcvm.2020.00074. PMID: 32478095; PMCID: PMC7240000.
- Tarantini, et. al., (2021). CCI, https://doi.org/10.1002/ccd.29674
- Iannaccone, et. al., (2022). International Journal of Cardiology, https://doi.org/10.1016/j.ijcard.2022.05.011.
- Shah, T., et. al., (2022). JSCAI, https://doi.org/10.1016/j.jscai.2021.100002
- Tehrani, B., et al., (2019). JACC, https://www.jacc.org/doi/10.1016/j.jacc.2018.12.084
- Toda, K., et. al., (2022). J Artif Organs, https://doi.org/10.1007/s10047-022-01328-1
IMP-352