Research Grants
Abiomed funds clinical research programs that investigate how mechanical circulatory support with Impella® heart pumps may improve patient outcomes.
All types of research programs will be considered for funding through Abiomed’s Investigator-Sponsored Studies (ISS) office. ISS are clinical studies that are initiated, developed, and conducted by a qualified researcher external to Abiomed who assumes all legal and regulatory responsibility for the trial. Potential ISS programs are funded through a competitive review process.
Areas of Interest
The following research domains have been identified by Abiomed to be areas of interest for innovation within the field. Applications within these domains will be given priority in the competitive application process. However, applications outside these domains will still be considered.
Best Practices in Urgent and Emergent Patient Populations
- Maximizing procedural, acute and long-term benefits while minimizing complication risks (ie. peripheral vascular complications and acute limb ischemia) for patients supported during urgent cases, including but not limited to: NSTEMI, CTO, peripartum cardiomyopathy and ECPR.
- Exploring methods to guide how Impella can minimize time to treatment and escalation in critical cardiac situations for improved patient outcomes.
- Exploring Alternatives to Heparin and A/C monitoring, including PPT and Anti-Xa guidance.
Impella Support for Heart Recovery
- Use of Impella 5.5® with SmartAssist® for reverse remodeling and recovery, including mechanisms of action, adjunctive therapies, right heart failure, outcomes, best practices for patient management and prediction of patient recoverability.
- Determining optimal duration of Impella support and weaning in Acute Myocardial Infarction with Cardiogenic Shock (AMI-CGS) and implications on both short-term and long-term patient recovery.
- Utilizing artificial intelligence to predict recovery and enhance decision-making processes in Impella-supported cases, focusing on AI Digitization to improve the prediction of patient outcomes.
Impella as a Platform to Enable Cardiac Interventions in High-Risk Populations
- Use of Impella in surgical and interventional procedures for pre-procedure optimization, BTT, BTR, or prevention of PCCS, including patient identification, timing of support, patient management.
End Organ Protection with Impella
- Identification of patients at risk for cardiorenal or other end organ dysfunction and unmasking the mechanisms of organ-to-heart crosstalk.
- Impact of Impella support on acute or chronic end organ dysfunction to improve patient outcomes.
Researchers interested in applying to Abiomed for competitive research funding should familiarize themselves with investigator expectations, Abiomed funding priorities, and the research grant review process.
Competitive Review Process
Abiomed carefully evaluates all submitted proposals. The investigator-sponsored studies review board evaluates applications based on the following criteria:
- Scientific and clinical impact
- Alignment with established funding priorities
- Innovation
- Study design and methodology
- Investigator experience & research site infrastructure
- Feasibility
- Patient safety
Abiomed and investigators funded through the ISS office share a common goal of generating high quality data, protecting human subjects, complying with all regulatory requirements, and advancing science for human health.
Special consideration will be given to applications investigating inequities and disparities observed in health outcomes.
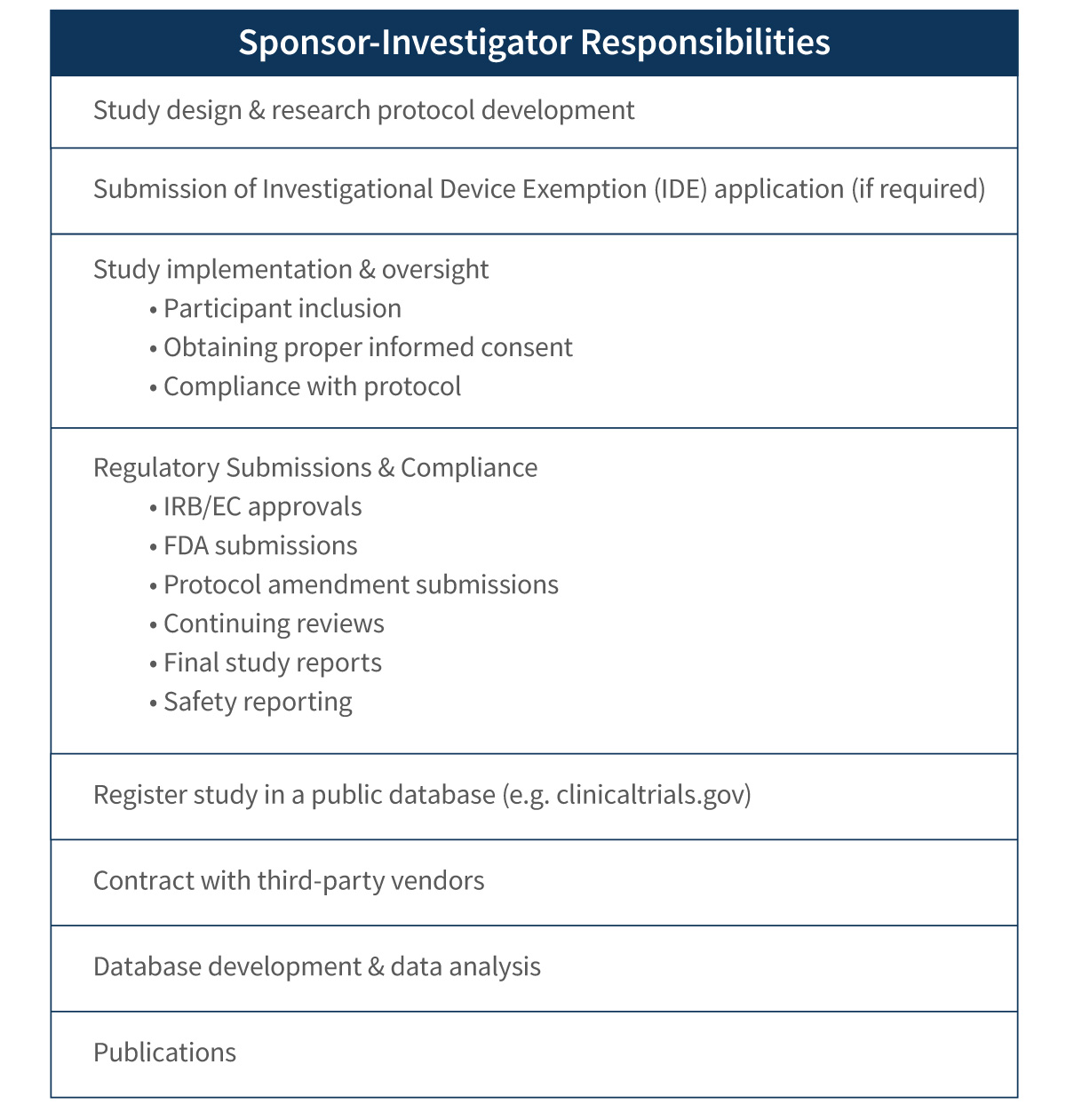
Investigator-Sponsored Studies Responsibilities
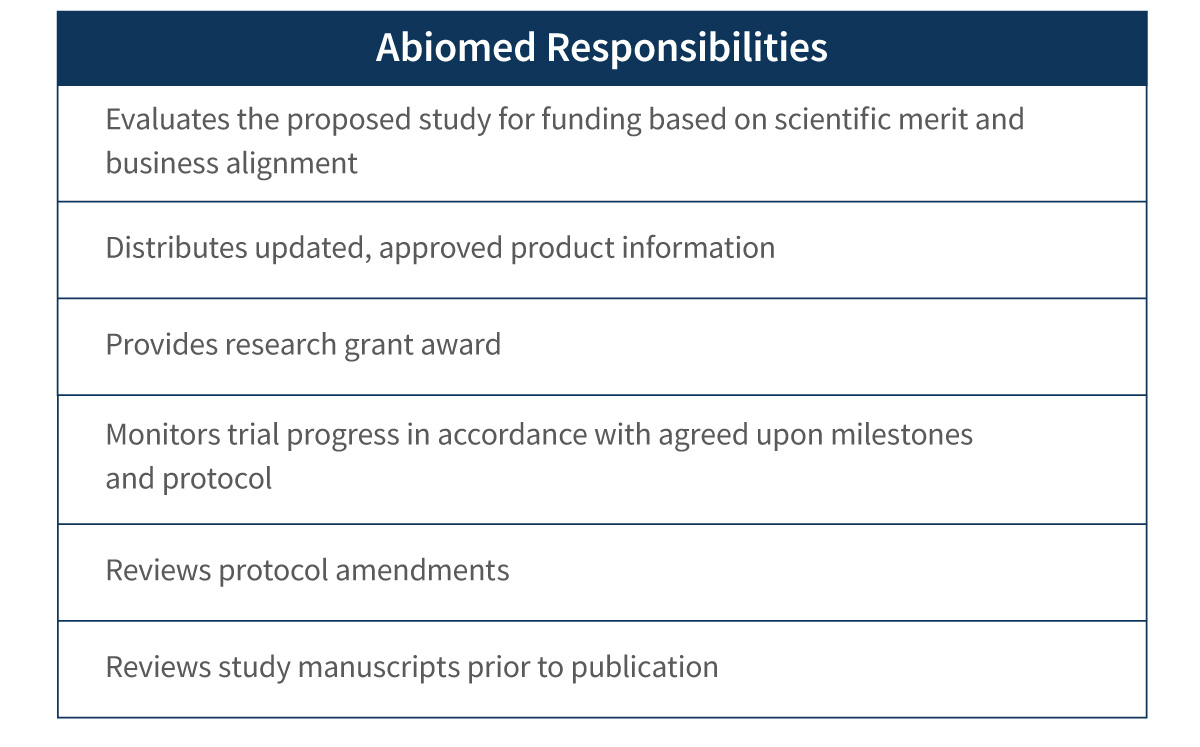
Investigator-Sponsored Study Application Process
1. Submit your complete application
You will be asked to submit an initial concept for review (Part A). If the initial concept is approved by Abiomed, a full study proposal (Part B) will be requested. You will be notified of your proposal status via email.
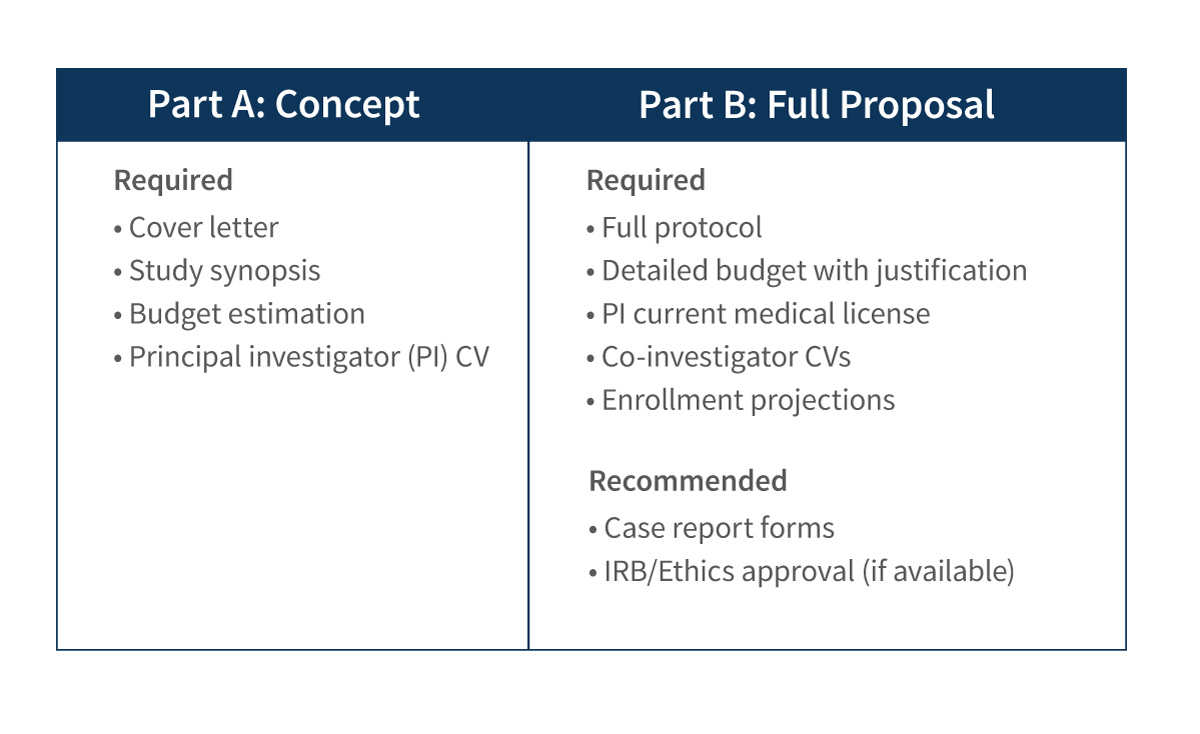
Please note, only complete applications will be considered for review. Proposals submitted by email will not be considered.
Grant Submission Templates
2. Proposal Review & Determination
Initial concept proposals are reviewed on an on-going basis, and full proposals are reviewed quarterly. You will be promptly notified of Abiomed’s grant funding decision via email. If you have any questions regarding the process, or would like to speak to the investigator-sponsored studies program team regarding your new or existing application, please contact us at [email protected].
Submission Deadlines
Part A: Concept
Part A submissions will be reviewed on an on-going basis
Part B: Full Proposal
March 3, 2025
May 19, 2025
August 25, 2025
November 17, 2025
NPS-2030
