Algorithms, Clinical Research & Data, Safety & Efficacy
FAQ: What Is the Clinical Evidence That Supports the Benefit of Placing the Impella® Heart Pump Pre-PCI?
A preponderance of evidence from the FDA cVAD Study®, Impella Quality (IQ) Database and physician-initiated National Cardiogenic Shock Initiative (NSCI) study support the recommendation that placing Impella 2.5® or Impella CP® pre-PCI improves outcomes.
Physicians are strongly encouraged to place Impella 2.5 or Impella CP prior to performing a PCI on patients in cardiogenic shock.
A critical mass of scientific research from six studies demonstrates that early implantation of Impella leads to the best outcomes. The real-world data is summarized in Figure 1.
How has this real-world evidence been validated?
Placing the Impella heart pump prior to revascularization is a best practice identified through analyses of data in the IQ Database, validated in the cVAD Study, and further validated by investigators leading the NCSI Study.
What does placing Impella pre-PCI allow for?
Placement of the Impella heart pump pre-PCI may allow for:
- Reperfusion of end organs prior to revascularization
- Hemodynamic support to the heart during revascularization
- The halting of progression of cardiogenic shock
Placement of Impella pre-PCI is included in multiple clinical protocols that demonstrate survival benefits, including the NCSI Study, which demonstrates that when best practices are followed, including placement of Impella pre-PCI, cardiogenic shock survival increases from ~50% to >70%.
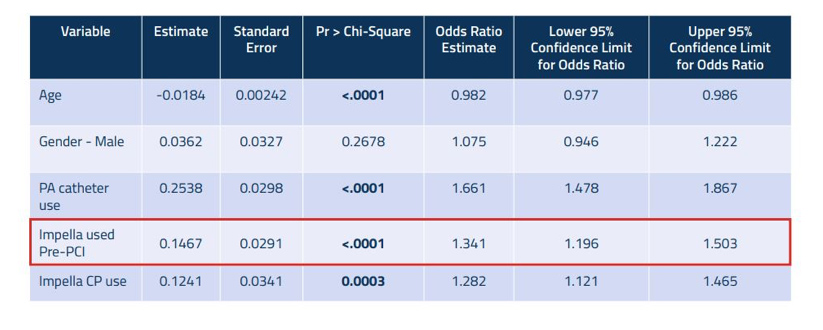
Figure 3: Predictors of Survival to Explant1
Physicians are strongly advised to place Impella 2.5 or Impella CP prior to revascularization.
References
1. O'Neill, W.W., et al. (2018). Am Heart J, 202, 33-38.
IMP-801